

In early development, each decision can determine whether your drug candidate moves forward or stalls.
For start-ups and biotech innovators, generating the right data at the right time is essential — not only for science, but also for securing funding and investor confidence.
At ERBC, we’ve designed Early Derisking Packages to help you make smart, confident go/no-go decisions before costly regulatory stages.
Our goal: transform uncertainty into insight — faster, smarter, and within your budget.
Early derisking is more than a safety check — it’s a strategic step in preclinical development.
It helps you:
With limited resources, early-stage innovators can’t afford to test everything — they need to test what matters most.
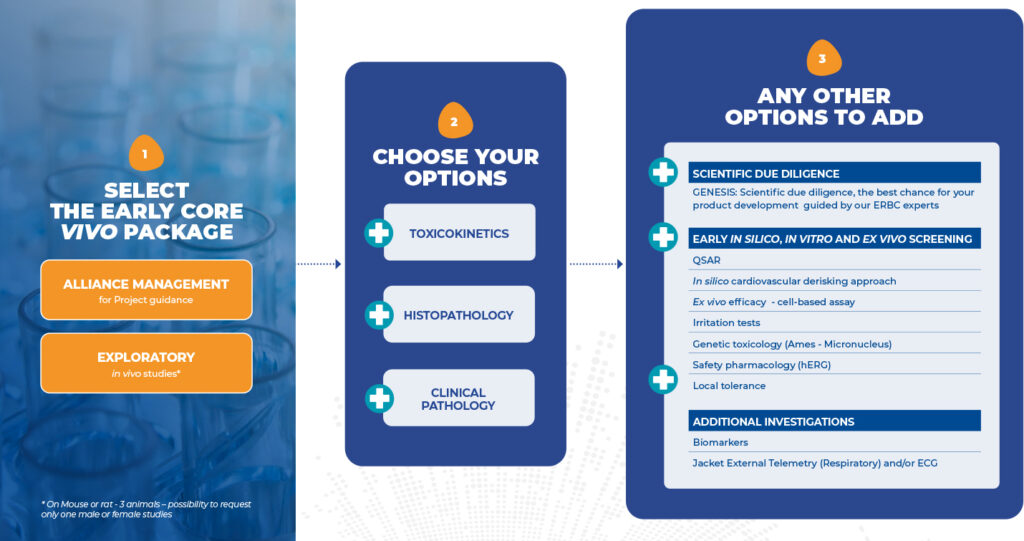
Our modular approach lets you select only what’s relevant to your science, timeline, and budget.
Each module is designed to provide decision-driving data, not just results.
Choosing ERBC means partnering with a one-stop preclinical CRO trusted by innovators across Europe.
We don’t just execute studies — we help you design the smartest strategy for your drug candidate.

Each discovery deserves the best chance to move forward.
ERBC’s Early Derisking Packages help you build a solid scientific foundation and present investors with credible, data-backed proof of concept.
Download our leaflet to explore the full offer.
Book a call with our scientific team to discuss your program.
Headquarters
ERBC Chemin de Montifault
18800 Baugy - France
+33 (0)2 48 23 00 23